Clinical microbiology plays a key role in infection prevention and control. Data from the clinical microbiology laboratory (CML) is the most common method for identifying healthcare-associated infections (HAIs). The role of the CML is to quickly and accurately detect pathogens and their antimicrobial resistance patterns.
Let’s take a look at the process.
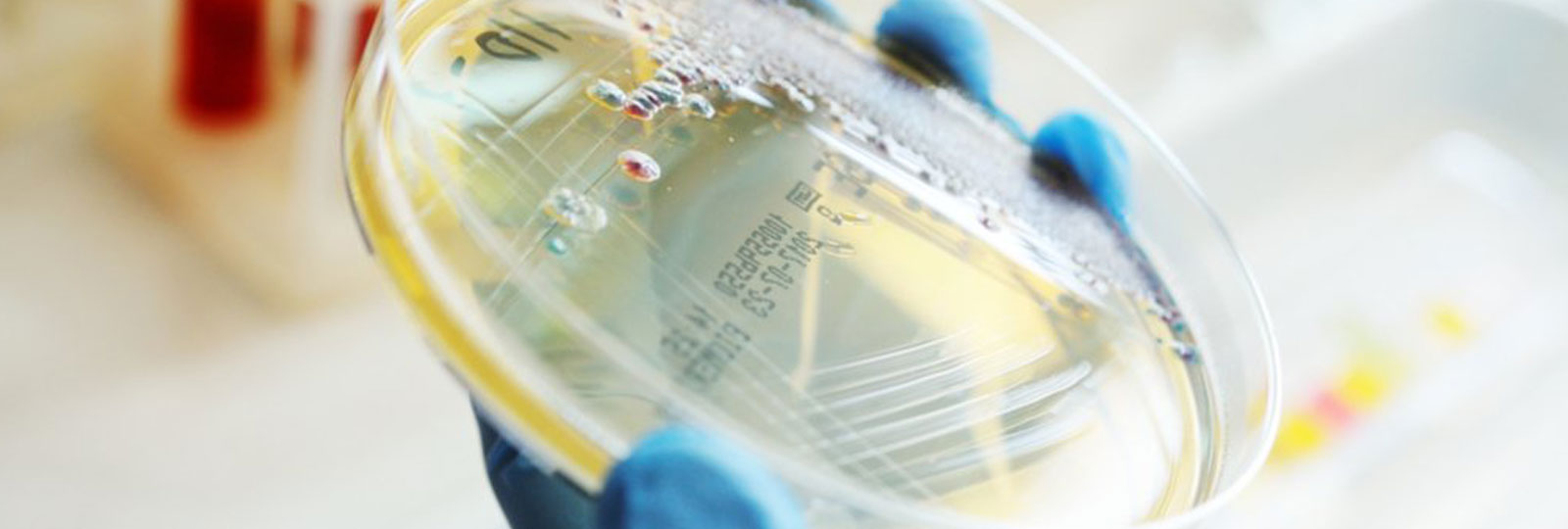
Growing bacteria
To isolate organisms within the laboratory, they have to be grown, or cultured, in a setting that mimics the normal conditions under which that cell would normally grow, whether in its host or in the environment.
The purpose of culturing bacteria in the laboratory is to grow a population of cells, known as a colony, that is 20-30 divisions of a single cell.
The rate of bacterial growth is dependent on several factors:
- Nutrients – water, oxygen, carbon dioxide, carbohydrates, iron, trace elements etc which are broken down by the bacteria and used an energy source
- Oxygen – certain types of bacteria grow better in an oxygen-rich environment while others prefer an oxygen-deprived environment
- Temperature – bacteria will cease to grow if the temperature drops below (or rises above) their optimum growth temperature. Pathogenic bacteria mostly grow at normal body temperature (37°C)
Processing a clinical specimen
The first stage in processing a clinical specimen is the inoculation of either a solid or liquid culture medium.
The most commonly collected clinical specimens are:
- Urine
- Sputum
- Wounds
- Faeces
- Rectal swabs
- Throat swabs
- Pernasal (nose) swabs
- Blood cultures
- Cerebrospinal fluid (CSF)
If using a solid culture:
A swab (inoculum) is rubbed over a quarter to one-third of the surface of a Petri dish containing a solid culture medium such as agar. A sterile wire is then used to ‘streak’ the inoculate over the surface of the medium. The lid of the Petri dish is replaced to avoid decontamination and the wire is ‘flamed’ in order to remove residual bacteria. The process is repeated with the original inoculate being spread into three sections, reducing with each spread. Bacteria that are separated from each other will grow as isolated colonies and can be assumed to have been formed by a single organism or organism cluster.
If using a liquid culture:
The nutrient broth will be inoculated from the plated specimen via an inoculating wire or loop. The specimen will then be incubated (aerobically or anaerobically) for 24-36 hours and will be observed for growth. The increased growth of bacteria will cause the nutrient broth to become cloudy and culture plates will normally grow a mix of bacteria so the colonies can be inoculated onto another plate containing a more selective culture medium. Bacteria grown in a liquid culture can also be inoculated onto a solid culture medium for identification.
Staining
Once an organism has been cultured it will need to be identified, as bacterial classification is key to determining the appropriate antibiotic therapy. Bacteria are naturally colourless, so gram staining is used to identify differences in the bacterial cell wall.
First, the specimen is transferred and heat is fixed to a glass slide, before being covered with blue dye (crystal violet) which is washed off after thirty-seconds and decolourised with acetone. The acetone is then removed and a red dye (safranin) is applied for one minute. The slide is then washed and blotted dry.
- If the bacteria are Gram-positive they will stain blue-black.
- If the bacteria are Gram-negative they will stain red-pink.
Mycobacteria cannot be gram-stained because of its high lipid content, and will instead need to be identified using the Ziehl-Neelson staining method. This is known as the “hot plate” method because it uses bright field microscopy.
Testing for antibiotic susceptibility and sensitivity
To test for antibiotic susceptibility, bacterial colonies are isolated from the culture plate and inoculated onto a new agar plate. Antibiotic-impregnated paper discs are then placed onto the plate and immediately begin to diffuse into the agar. Bacteria that are susceptible to antibiotics will form a zone of inhibition around the disc. Those that are resistant will grow right up to the edge.
Typing
‘Typing’ is used to determine the species of bacteria and to differentiate between different strains. This is carried out in a specialist Public Health England Reference Laboratory. Typing techniques can also be used to determine the evolution of the strain and any emerging pathogens or clones that could represent a further risk.
HAIs remain one of the biggest challenges facing health services around the world. The application of infection prevention and control in hospitals is crucial to the effective management of patient care and reducing the incidence of HCAIs. Clinical microbiology and the CML play a critical part in this.





